The Basics of Batteries: What Is Battery

A battery is a device that stores chemical energy and converts it into electrical energy. This process involves a chemical reaction that releases electrons, creating an electric current. Imagine a tiny factory where chemical reactions create electricity!
Components of a Battery
Batteries consist of several essential components that work together to generate and store energy. These components are:
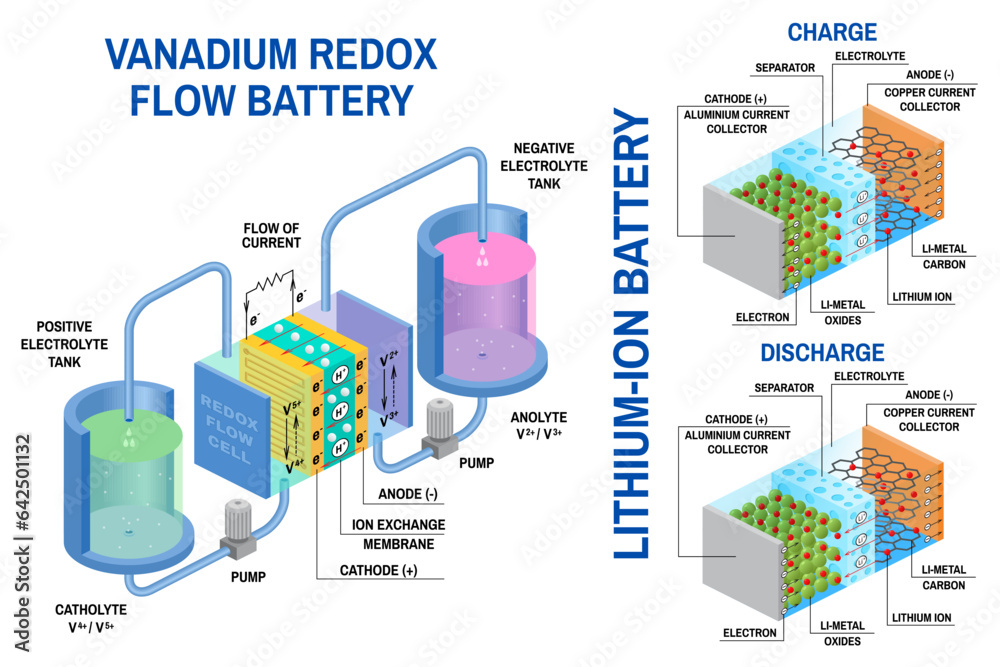
- Anode: The negative electrode of a battery. It releases electrons during the chemical reaction, creating the flow of electricity.
- Cathode: The positive electrode of a battery. It receives electrons during the chemical reaction.
- Electrolyte: A conductive solution or paste that allows the flow of ions between the anode and cathode. It’s like a bridge connecting the two electrodes.
- Separator: A thin membrane that physically separates the anode and cathode, preventing direct contact but allowing ion flow. This prevents short circuits and ensures the battery operates safely.
Chemical Reactions in Batteries
The heart of a battery’s operation lies in the chemical reactions occurring within it. These reactions involve the movement of electrons and ions, creating an electrical potential difference. The type of chemical reaction determines the battery’s voltage and energy storage capacity.
The most common type of battery uses a redox reaction, where electrons are transferred between chemical species, resulting in a change in oxidation states.
Everyday Devices Powered by Batteries, What is battery
Batteries power countless devices we use daily, from small gadgets to large appliances. Here are a few examples:
- Mobile phones: These devices rely on rechargeable lithium-ion batteries to power their various functions, including calls, texting, and internet browsing.
- Laptops: Similar to mobile phones, laptops use rechargeable lithium-ion batteries to provide power for extended use without needing a direct power source.
- Electric vehicles: These vehicles use large, high-capacity batteries to store energy for long-distance travel. The battery packs in electric vehicles are typically made up of multiple smaller battery cells connected together.
- Remote controls: These devices use small, disposable batteries, often alkaline batteries, to power their functions. They are used to control TVs, stereos, and other electronic devices.
Types of Batteries
Batteries are electrochemical energy storage devices that convert chemical energy into electrical energy. They are essential components in a wide range of applications, from powering portable electronics to providing backup power for critical systems. Different types of batteries are designed for specific purposes and exhibit varying characteristics in terms of energy density, power output, lifespan, and cost.
Battery Types and Their Characteristics
Different types of batteries are classified based on their electrochemical composition and the materials used in their construction. Each battery type has unique characteristics that make it suitable for specific applications.
- Lithium-ion (Li-ion) Batteries: Lithium-ion batteries are widely used in portable electronics, electric vehicles, and energy storage systems. They offer high energy density, meaning they can store a large amount of energy in a small package. They also have a relatively long lifespan and can withstand numerous charge-discharge cycles.
- Lead-acid Batteries: Lead-acid batteries are the oldest and most common type of battery. They are relatively inexpensive and have a high power output, making them suitable for applications like car batteries and backup power systems. However, they are heavy and have a limited lifespan compared to other battery types.
- Alkaline Batteries: Alkaline batteries are primary batteries, meaning they are designed for single use and cannot be recharged. They are commonly used in everyday devices like remote controls, toys, and flashlights. Alkaline batteries offer good energy density and a long shelf life.
- Nickel-cadmium (NiCd) Batteries: Nickel-cadmium batteries are rechargeable batteries that have a high power output and are known for their durability. However, they have a memory effect, meaning they can lose capacity if they are not fully discharged before charging. They also contain cadmium, a toxic metal, which raises environmental concerns.
Comparison of Battery Types
| Battery Type | Characteristics | Applications | Advantages | Disadvantages |
|---|---|---|---|---|
| Lithium-ion (Li-ion) | High energy density, long lifespan, high power output, relatively expensive | Portable electronics, electric vehicles, energy storage systems | High energy density, long lifespan, high power output | Relatively expensive, safety concerns, susceptible to overheating |
| Lead-acid | Low cost, high power output, heavy, limited lifespan | Car batteries, backup power systems | Low cost, high power output | Heavy, limited lifespan, environmental concerns |
| Alkaline | Primary battery, good energy density, long shelf life | Everyday devices, remote controls, toys, flashlights | Good energy density, long shelf life, low cost | Not rechargeable, limited lifespan |
| Nickel-cadmium (NiCd) | Rechargeable, high power output, durable, memory effect, environmental concerns | Power tools, cordless phones, medical devices | High power output, durable | Memory effect, environmental concerns |
Internal Structure of Battery Types
Lithium-ion Battery
What is battery –
A typical lithium-ion battery consists of a positive electrode (cathode), a negative electrode (anode), a separator, and an electrolyte. The cathode is typically made of lithium cobalt oxide (LiCoO2), while the anode is made of graphite. The separator is a porous membrane that prevents the electrodes from coming into contact, while the electrolyte is a liquid or solid that allows lithium ions to move between the electrodes. During charging, lithium ions move from the cathode to the anode, storing energy. During discharge, lithium ions move back to the cathode, releasing energy.
Lead-acid Battery
A lead-acid battery consists of lead plates immersed in a sulfuric acid electrolyte. The positive plates are made of lead dioxide (PbO2), while the negative plates are made of lead (Pb). During charging, the sulfuric acid reacts with the lead plates to form lead sulfate (PbSO4) and water. During discharge, the lead sulfate reacts with the sulfuric acid to form lead and lead dioxide, releasing energy.
Alkaline Battery
An alkaline battery consists of a zinc anode, a manganese dioxide cathode, and an alkaline electrolyte. The zinc anode is oxidized during discharge, releasing electrons that flow through an external circuit to the manganese dioxide cathode. The manganese dioxide is reduced, and the reaction produces water and zinc oxide.
Nickel-cadmium Battery
A nickel-cadmium battery consists of a nickel hydroxide cathode, a cadmium anode, and a potassium hydroxide electrolyte. During charging, the nickel hydroxide is reduced to nickel oxide hydroxide, while the cadmium anode is oxidized to cadmium hydroxide. During discharge, the nickel oxide hydroxide is oxidized back to nickel hydroxide, while the cadmium hydroxide is reduced to cadmium, releasing energy.
Battery Performance and Characteristics

Understanding how a battery performs is crucial for choosing the right battery for a specific application. Key performance metrics provide insights into a battery’s capabilities and limitations.
Battery Performance Metrics
Battery performance metrics quantify how well a battery functions. These metrics are essential for comparing different battery types and selecting the most suitable one for a particular application.
- Capacity: This refers to the amount of electrical charge a battery can store. It is measured in Ampere-hours (Ah) or milliampere-hours (mAh). A higher capacity indicates a battery can deliver power for a longer duration.
- Voltage: This is the electrical potential difference between the battery’s terminals. It is measured in Volts (V). The voltage determines the amount of energy delivered per unit of charge.
- Current: This is the rate at which electrical charge flows through the battery. It is measured in Amperes (A). A higher current indicates a faster rate of charge delivery, typically associated with higher power applications.
- Energy Density: This measures the amount of energy stored per unit of mass or volume. It is typically expressed in Watt-hours per kilogram (Wh/kg) or Watt-hours per liter (Wh/L). A higher energy density indicates a battery can store more energy in a smaller package.
- Cycle Life: This refers to the number of charge-discharge cycles a battery can endure before its capacity significantly degrades. It is typically expressed in the number of cycles or years of operation. A longer cycle life indicates a battery can withstand repeated charging and discharging without significant performance degradation.
Factors Influencing Battery Performance and Lifespan
Several factors can affect a battery’s performance and lifespan, impacting its overall effectiveness. Understanding these factors allows for optimizing battery usage and extending its service life.
- Temperature: Batteries operate most efficiently within a specific temperature range. Extreme temperatures, both high and low, can negatively impact performance and shorten lifespan. High temperatures can accelerate chemical reactions within the battery, leading to faster degradation. Low temperatures can reduce the battery’s internal resistance, decreasing its capacity and power output.
- Charge/Discharge Rate: The rate at which a battery is charged or discharged also affects its performance. Fast charging or discharging can generate heat and stress the battery, leading to faster degradation. Conversely, slow charging and discharging can extend the battery’s lifespan.
- Depth of Discharge (DoD): The DoD refers to the percentage of the battery’s capacity that is discharged during a cycle. Deep discharges can stress the battery and shorten its lifespan. Keeping the DoD low can extend the battery’s life by minimizing the stress on the battery’s internal components.
- Age: Batteries naturally degrade over time, even if not used. This degradation is due to internal chemical reactions and the gradual loss of active materials. The rate of degradation can vary depending on the battery type, usage patterns, and environmental conditions.
Battery Performance Degradation with Time
The performance of a battery can degrade over time due to various factors. This degradation is often characterized by a decrease in capacity, voltage, and cycle life.
| Factor | Effect on Battery Performance |
|---|---|
| Temperature | Higher temperatures accelerate degradation, while lower temperatures decrease capacity and power output. |
| Charge/Discharge Rate | Fast charging or discharging can generate heat and stress the battery, leading to faster degradation. |
| Depth of Discharge (DoD) | Deep discharges can stress the battery and shorten its lifespan. |
| Age | Batteries naturally degrade over time, leading to a decrease in capacity, voltage, and cycle life. |
“Battery performance is a complex interplay of factors. Understanding these factors is crucial for optimizing battery usage and extending its service life.”
Battery, in legal terms, is an intentional act that causes harmful or offensive contact with another person. This can range from a simple shove to a more serious assault. The recent news regarding skai jackson arrested highlights the importance of understanding the legal definition of battery, as it is a criminal offense that can carry significant consequences.
It’s crucial to remember that any form of unwanted physical contact can constitute battery, and it is essential to respect the boundaries and personal space of others.
A battery is a device that converts chemical energy into electrical energy. This process is achieved through a chemical reaction that occurs within the battery, resulting in the movement of electrons. While the concept of a battery is straightforward, the news cycle can be quite unpredictable, as exemplified by the recent reports on skai jackson pregnant tmz.
This unexpected news highlights how the world of information can be as dynamic and complex as the intricate chemical reactions within a battery.